
The World Health Organization (WHO) periodically reviews all malaria RDTs manufactured for diagnostic use and sets the limit of detection for these tests at 200 parasites per μL. 3ĭespite the many advantages of RDTs, the changing climate of infectious disease education, prevention, and treatment has highlighted the need for improved tests. The predominant RDT biomarker indicative of Plasmodium falciparum infection is Histidine Rich Protein II ( pfHRPII), while Plasmodium lactate dehydrogenase ( pLDH) serves as a pan-specific biomarker. 3 These RDTs detect protein biomarkers of the malarial parasite. 6 In 2006, over 16 million RDTs were delivered to underdeveloped nations for the detection of malaria alone. 5 Additionally, these tests have been widely used in public health programs to aid with patient management, disease surveillance and treatment campaigns.
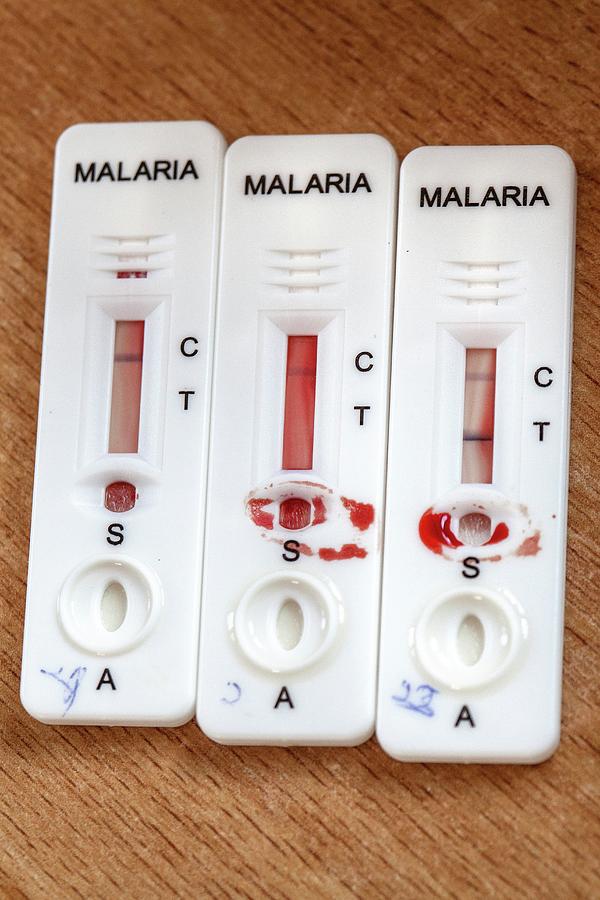
3,4 Several advantages of RDTs include low-cost, rapid time to result, and ease of use and interpretability. Lateral flow immunochromatographic rapid diagnostic tests (RDTs), which operate much like a commercial pregnancy test, were developed to circumvent these challenges and bring affordable disease diagnosis to low-resource areas. 2 Light microscopy is currently the gold standard for blood-borne disease detection, but intermittent access to functional microscopes and trained microscopists prevents its widespread use at the point of care. Molecular based diagnostic methods such as enzyme immunoassays (ELISA) and quantitative polymerase chain reaction (qPCR) are powerful tools for the detection of protein and nucleic acid biomarkers of infectious diseases, but reagent instability and cost of these assays in addition to time and expertise needed to perform the test limits their use in underdeveloped areas.

1 These underdeveloped, remote areas are often characterized by poverty, absent or intermittent electricity, hot and humid environmental conditions as well as a lack of skilled clinicians. Introduction The advent of point of care (POC) diagnostic tools has changed the face of healthcare in nations affected by the ongoing spread of infectious diseases. Finally, through individual donor samples, the correlations between donor source, WHO panel detection scores and RDT signal intensities were explored.
For the best performing RDTs the limits of detection were found to be as low as 3 parasites per μL. This lowered the limit of detection for RDT brands to submicroscopic parasitemias. Greater than a 4-fold RDT signal enhancement was observed as a result of the sample processing step. In this study, we demonstrate enhancement in the performance of six RDT brands when a simple sample-processing step is added to the front of the diagnostic process.
In order for eradication of the disease to be achieved, this problem must be solved. Unfortunately, many of these tests do not detect asymptomatic malaria carriers. The prospect of overcoming the problems associated with current RDTs with a new generation of alternative malaria antigen targets is also described.Lateral flow immunochromatographic rapid diagnostic tests (RDTs) are the primary form of medical diagnostic used for malaria in underdeveloped nations. The difficulties associated with RDTs, such as genetic variability in the Pfhrp2 gene and the persistence of antigens in the bloodstream following the elimination of parasites, are discussed. This paper describes recent developments in malaria RDTs, reviewing RDTs detecting PfHRP2, pLDH and plasmodial aldolase. However, the specificities, sensitivities, numbers of false positives, numbers of false negatives and temperature tolerances of these tests vary considerably, illustrating the difficulties and challenges facing current RDTs. Tests targeting HRP2 contribute to more than 90% of the malaria RDTs in current use. Three antigens - Plasmodium falciparum histidine-rich protein 2 (PfHRP2), plasmodial aldolase and plasmodial lactate dehydrogenase (pLDH) - are currently used for RDTs. In the last decade, there has been an upsurge of interest in developing malaria rapid diagnostic test (RDT) kits for the detection of Plasmodium species.


 0 kommentar(er)
0 kommentar(er)
